Covid-19 Variants a Global Headache
The Oxford-AstraZeneca vaccines roll-out is underway in Kenya after the arrival of the first batch. Scientists and top health ministry officials are optimistic about its efficacy despite reports of Covid-19 variants in Kenya.
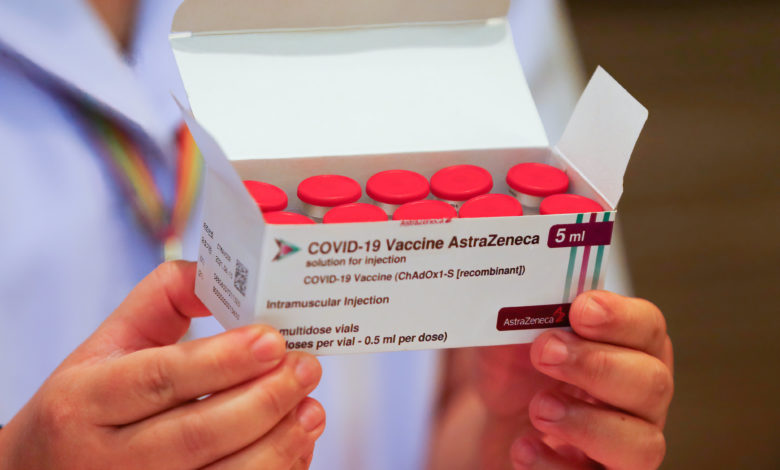
On March 3, 2021, the first 1.02 million doses of the vaccine were flown into the country. They are to be used in its first phase of immunization against the viral infection.
It is however yet to be seen whether the state officials’ confidence in the vaccines will translate into increased trust by skeptical sections of the public.
A study published by Trends and Insights for Africa (TIFA) in December 2020 indicates that only 54 percent of Kenyans were confident that the vaccine would be safe and effective.
According to the research institution, almost half of the country’s residents are uncomfortable with the idea of taking the shot. This will most likely pose a headache to Kenya’s efforts to vaccinate at least 30 percent of the population.
Uncertainty ahead
The country’s efforts could also be hampered by a number of factors. This includes the availability of vaccines doses for the next two phases and vaccine hesitancy due to misconceptions on the jab.
Dr Willis Akhwale, the Covid-19 Vaccine Board Director, said that the government got the vaccines for free. The country will however spend at least Sh34 billion to immunize 30 per cent of its population from March 2021 to June 2023.
The first phase of the vaccination plan targets 1.25 million people. Phase two targets 9.76 million individuals above the age of 50 years and those with underlying conditions or comorbidity.
Kenya requires 30 million doses to vaccinate 60 per cent of its population as recommended by the Global Alliance for Vaccines (Gavi).
Some experts believe variants of the corona virus could impair the effectiveness of Covid-19 vaccines. A variant is a version of the virus that has accumulated enough mutations to represent a separate branch on the family tree.
By March 5, 2021, Kenya’s Covid-19 caseload was past the 106,000 mark and nearly 1,900 deaths had been reported.
Existing variants
Dr Rudi Eggers, the World Health Organization (WHO) country representative for Kenya has termed the variants a global concern.
“As far as we can say at the moment, the vaccines will be effective, but these are still early days and we will have to do more research on that. Early indications show that the available vaccines are still effective against the virus strain but it is possible there will be a decrease in the effectiveness of the vaccine,” said Dr Eggers.
On January 21, 2021, Acting Director General of Health Dr Patrick Amoth, confirmed that two cases of the new variant had already been recorded in Kenya.
In January, 2021 the Kenya Medical Research Institute (Kemri), which is the country’s premier medical research facility, had projected a spike in infections in March 2021.
In December 2020, the first of the now rapidly spreading new virus strains was first described by the United Kingdom.
South African variant
On December 18, 2020, another variant was discovered. The so called South African strain first detected on December 18, 2020, was named 501.V2 due to the mutation they found in the protein that the virus uses to invade human cells. It has been rapidly spreading through the UK to other countries. More updates on the variants so far discovered worldwide are contained in the Global Variant Reports.
In Kenya, a 10 member Kemri research team successfully isolated the South African variant from samples taken from Taita Taveta region, in the coastal part of Kenya. They announced the outcome in January 2021.
The discovery was just the latest in a list of 16 variants that have so far been detected in the country, bringing the total in circulation to 20.
Variants in Kenya
Scientists used genetic data drawn from the nasal samples of 205 people from eight counties namely: Taita Taveta, Tana River, Lamu, Kilifi, Mombasa, Kajiado, Nairobi and Kwale counties between June and October 2020.
Charles Agoti, Principal researcher and investigator at Kemri said it could lead to more Covid-19 cases in the country.
“Our interpretation is that this one place (Taita Taveta) in Kenya represents the majority of the sequenced samples. It imply that it could be more contagious or transmissible, and result in a rise in local cases,” said Agoti.
Genomic sequencing analyses the virus sample taken from a diagnosed patient and compares it with other cases.
In a development that could complicate the fight against the pandemic, the new strain can spread faster, and causes more severe cause of disease.
Dr Agoti however indicated that the variant would not significantly affect the ability of the available vaccines to protect vaccinated individuals against infection.
No need to panic
Scientists still lack comprehensive knowledge on how widely these new variants have spread. The samples need to be sequenced which not many laboratories can do.
They are also yet to understand precisely how the disease caused by these new variants differ from other variants reported in circulation.
According to Robert Bollinger, a professor of Infectious Diseases at Johns Hopkins School of Medicine, in the USA ,some of the mutations in the B.1.1.7 variant seem to affect the coronavirus’s spike protein.
The proteins help the virus bind itself to our cells in the nose, lungs and other parts of the body. Prof Bollinger says preliminary studies on the variants indicate that they are more contagious than the previous ones.
Containing the spread
Infectious disease experts are studying these variants to find a fast and effective method of containing their spread. They want to find out how contagious the various variants are.
From the findings they can update the vaccines to be more effective against new variants of the respiratory infection. As the vaccines continue to be rolled out across the world, concern is rising over the emergence of the new variant in Africa.
Kenya benefitted from the February 15, 2021 WHO listing of the Oxford-AstraZeneca vaccine that authorized it for emergency use.
Oxford-AstraZeneca has had its share of controversy. On February 7, 2021, South Africa dropped it from the national Covid-19 vaccine programme.
The move was attributed to studies by the Witwatersrand University’s Vaccines and Infectious Diseases Analytics (VIDA) Research Unit, which is responsible for the Oxford Covid-19 vaccines trials in South Africa, showing that it does not work well against the South African variant.
The pharmaceutical giant has however confirmed it is tweaking the vaccine to enable it to cope with the variant mutations. The new vaccine versions could however take up to 6 months to be ready for use.
Involvement of Africa
Experts have previously called for greater involvement by African countries in Covid-19 vaccine trials, as a way for the continent to gain maximum price and research benefits for participating countries.
Dr Borna Nyaoke Anoke is the Clinical Trials Manager at the Drugs for Neglected Diseases Initiative/Global Antibiotic Research and Development Partnership (DNDI/GARDP). At a Webinar organized by Media for Environment, Science, Health and Agriculture (Mesha), she asked African states not to shy away from clinical trials.
She said it would result in cheaper vaccines and gaining of valuable research insight into the vaccine development process.
She added that countries that skipped vaccine trials would probably end up buying them at higher prices once they are approved for use.
“It does not make economic sense for pharmaceutical firms in the north that includes Europe and America, to test and develop a vaccine in their home countries at very high costs and then sell it here at a price similar to the one in their home countries. It is even harder to expect them to give discounted rates on the vaccines to African countries,” she said.
According to Dr Nyaoke Anoke, pharmaceutical firms prefer Africa for medicine and vaccine trials purely for scientific and not racist reasons.
“Africa has the richest genetic variety and this makes it an ideal location for clinical trials involving vaccine and drug candidates,” she told Sayansi Magazine which is published by Mesha.
Africa still lags behind in Covid-19 vaccine research and development, with a recent study showing that only 4 percent of published research is relevant to the continent.
What journalists should do:
- Monitor the vaccine distribution process to check whether it is being done on schedule and using the laid down guidelines.
- Ask for a comprehensive clarification from the government on which Covid-19 variants have been discovered in the country, as well as the measures put in place to protect Kenyans from infection.
- Ask for the list of priority and find out when the vaccine will be given to wider the wider public, and whether the criteria for administration is being followed.
- Alert the public on the safety and efficacy concerns around the vaccination program as explained by experts.
- Dispel rumours and myths around the vaccines by sharing facts.
- Check our LIST OF EXPERTSfor this and other topics.
greatest content